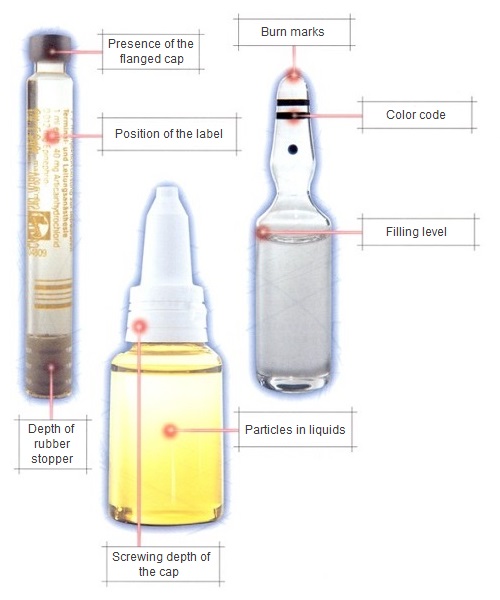
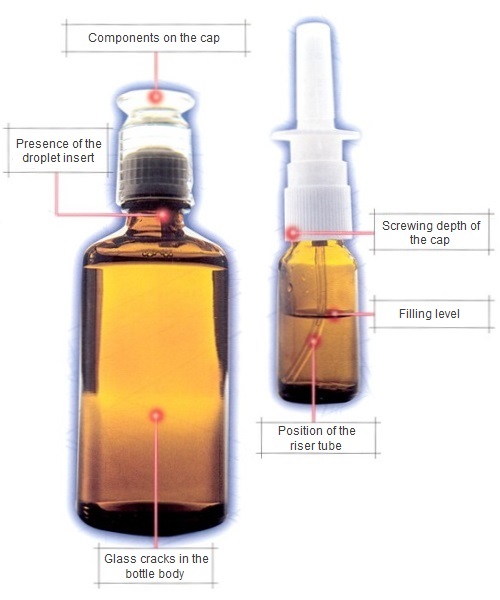
A world without medicines is no longer imaginable today. The regulations in Germany, based on the German Medicines Act (source: Federal Office of Justice), make strict quality and safety controls indispensable.
With image processing based inspection and identification solutions (ID), defective pharmaceutical products can be easily and reliably identified or faulty barcodes or label texts avoided. This leads to increased productivity, increased yield and helps to permanently achieve the set quality targets. In addition, it can be guaranteed that health products produced meet all requirements with regard to patient safety and traceability.

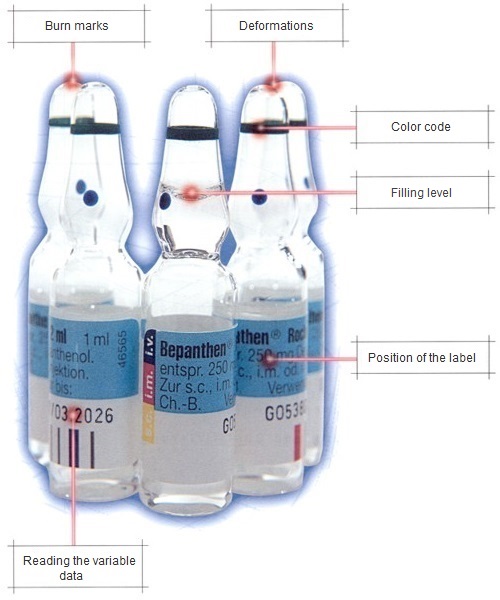
Our inspection systems use fully automatic vision sensors or special vision systems systems, which can be operated comfortably and easily via our KUPvision software. Beside the mentioned inspection systems, Krempien+Petersen Qualitäts-Kontrollsysteme GmbH also offers the customized development of software and hardware.
Possible fields of application
- Checking the correctness and position of labels
- Fill level inspection of vials, ampoules, bottle packs, etc.
- Blister controls for tablets
- OCV, OCR readings on different products and surfaces
- 1D and 2D code readings on different products and surfaces
- Presence inspections of different products
- Glass breakage inspections for vials, ampoules, etc.
- Particle inspection systems on vials, ampoules, etc.
- Color inspection on ampoules
- Surface inspections on different products
- Check for contamination on different products
- Color inspection of caps on vials
- Print quality inspections on different surfaces
- Flared cap inspections on vials